Valige keel:
ST seadmed & Technology OÜ (STET) tribo-elektrostaatilise vöö eraldaja is ideally suited for beneficiating very fine (<1m) mõõdukalt jäme (500m) mineral particles, väga suure läbilaskevõimega. Experimental findings demonstrated the capability of the STET separator to beneficiate bauxite samples by increasing available alumina while simultaneously reducing reactive and total silica. STET technology is presented as a method to upgrade and pre-concentrate bauxite deposits for use in alumina production. Dry processing with the STET separator will result in a reduction in operating costs of refinery due to lower consumption of caustic soda, savings in energy due to lower volume of inert oxides and a reduction in volume of alumina refinery residues (ARR or red mud). Lisaks, the STET technology may offer alumina refiners other benefits including increased quarry reserves, extension of red mud disposal site life, and extended operating life of existing bauxite mines by improving quarry utilization and maximizing recovery. The water-free and chemical-free by-product produced by the STET process is usable for manufacture of cement in high volumes without pre-treatment, erinevalt punasest mudast, mille kasulik taaskasutamine on piiratud.
1.0 Sissejuhatus
Aluminum production is of central importance for the mining and metallurgy industry and fundamental for a variety of industries [1-2]. While aluminum is the most common metallic element found on earth, in total about 8% of the Earth’s crust, as an element it is reactive and therefore does not occur naturally [3]. Hence, aluminum-rich ore needs to be refined to produce alumina and aluminum, resulting in significant generation of residues [4]. As the quality of bauxite deposits globally decline, the generation of residue increases, posing challenges to the alumina and aluminum-making industry in terms of processing costs, costs of disposal and the impact on the environment [3].
Alumiiniumi rafineerimise peamine lähtematerjal on boksiit, the world’s main commercial source of aluminum [5]. Bauxite is an enriched aluminum hydroxide sedimentary rock, produced from the laterization and weathering of rocks rich in iron oxides, aluminum oxides, or both commonly containing quartz and clays like kaolin [3,6]. Bauxite rocks consists mostly of the aluminum minerals gibbsite (Al(OH)3), boehmite (γ-AlO(OH)) and diaspore (α-AlO(OH)) (Tabel 1), and is usually mixed with the two iron oxides goethite (FeO(OH)) and hematite (Fe2O3), the aluminum clay mineral kaolinite, small amounts of anatase and/or titania (TiO2), ilmenite (FeTiO3) and other impurities in minor or trace amounts [3,6,7].
Termineid trihüdraat ja monohüdraat kasutavad tööstus tavaliselt erinevat tüüpi boksiitide eristamiseks.. Bauxite'i, mis on täielikult või peaaegu kogu gibbsite laager, nimetatakse trihüdraadi maagiks; kui domineerivateks mineraalideks on boehmiit või diaspore, nimetatakse seda monohüdraadimaagiks [3]. Gibbsite ja boehmite segud on tavalised igat tüüpi boksiitides, boehmiit ja diaspore harvem, ja gibbsite ja diaspore haruldane. Each type of bauxite ore presents its own challenges in terms of mineral processing and beneficiation for the generation of alumina [7,8].
Tabel 1. Chemical composition of Gibbsite, Boehmite and Diaspore [3].
| Chemical Composition | Gibbsite AL(OH)3 or Al2O3.3H2O | Boehmite ALO(OH) or Al2O3.H2O | Diaspore ALO(OH) or Al2O3.H2O |
|---|---|---|---|
| Al2O3 wt% | 65.35 | 84.97 | 84.98 |
| (OH) wt% | 34.65 | 15.03 | 15.02 |
Bauxite deposits are spread worldwide, mostly occurring in tropical or subtropical regions [8]. Bauxite mining of both metallurgical and non-metallurgical grade ores is analogous to the mining of other industrial minerals. Normally, the beneficiation or treatment of bauxite is limited to crushing, sieving, washing, and drying of the crude ore [3]. Flotation has been employed for the upgrading of certain low-grade bauxite ores, however it has not proven highly selective at rejecting kaolinite, a major source of reactive silica especially in trihydrate bauxites [9].
The bulk of bauxite produced in the world is used as feed for manufacturing of alumina via the Bayer process, a wet-chemical caustic-leach method in which the Al_2 O_3 is dissolved out of the bauxite rock by using a caustic soda rich solution at elevated temperature and pressure [3,10,11]. Subsequently, the bulk of alumina is utilized as feed for the production of aluminum metal via the Hall-Héroult process, which involves electrolytic reduction of alumina in a bath of cryolite (Na3AlF6). It takes about 4-6 tons of dried bauxite to produce 2 t of alumina, which in turns yields 1 t of aluminum metal [3,11].
The Bayer process is initiated by mixing washed and finely ground bauxite with the leach solution. The resulting slurry containing 40-50% solids is then pressurized and heated with steam. At this step some of the alumina is dissolved and forms soluble sodium aluminate (NaAlO2), but due to the presence of reactive silica, a complex sodium aluminum silicate also precipitates which represents a loss of both alumina and soda. The resulting slurry is washed, and the residue generated (st, red mud) is decanted. Sodium aluminate is then precipitated out as aluminum trihydrate (Al(OH)3) through a seeding process. The resulting caustic soda solution is recirculated into the leach solution. Lõpuks, the filtered and washed solid alumina trihydrate is fired or calcined to produce alumina [3,11].
Leaching temperatures may range from 105°C to 290°C and corresponding pressures range from 390 kPa to 1500 kPa. Lower temperatures ranges are used for bauxite in which nearly all the available alumina is present as gibbsite. The higher temperatures are required to digedepositsst bauxite having a large percentage of boehmite and diaspore. At temperatures of 140°C or less only gibbsite and kaolin groups are soluble in the caustic soda liquor and therefore such temperature is preferred for the processing of trihydrate alumina . At temperatures greater than 180°C alumina present as trihydrate and monohydrate are recoverable in solution and both clays and free quartz become reactive [3]. Operating conditions such as temperature, pressure and reagent dosage are influenced by the type of bauxite and therefore each alumina refinery is tailored to a specific type of bauxite ore. The loss of expensive caustic soda (NaOH) and the generation of red mud are both related to the quality of bauxite used in the refining process. Üldiselt, the lower the Al_2 O_3 content of bauxite, the larger the volume of red mud that will be generated, as the non-Al_2 O_3 phases are rejected as red mud. Lisaks, the higher the kaolinite or reactive silica content of bauxite, the more red mud will be generated [3,8].
High-grade bauxite contains up to 61% Al_2 O_3, and many operating bauxite deposits -typically referred as non-metallurgical grade- are well below this, occasionally as low as 30-50% Al_2 O_3. Because the desired product is a high purity
Al_2 O_3, the remaining oxides in the bauxite (Fe2O3, SiO2, TiO2, organic material) are separated from the Al_2 O_3 and rejected as alumina refinery residues (ARR) or red mud via the Bayer process. Üldiselt, the lower quality the bauxite (st, lower Al_2 O_3 content) the more red mud that is generated per ton of alumina product. Lisaks, even some Al_2 O_3 bearing minerals, notably kaolinite, produce undesirable side reactions during the refining process and lead to an increase in red mud generation, as well as a loss of expensive caustic soda chemical, a large variable cost in the bauxite refining process [3,6,8].
Red mud or ARR represents a large and on-going challenge for the aluminum industry [12-14]. Red mud contains significant residual caustic chemical leftover from the refining process, and is highly alkaline, often with a pH of 10 – 13 [15]. It is generated in large volumes worldwide – according to the USGS, estimated global alumina production was 121 million tons in 2016 [16]. This resulted in an estimated 150 million tons of red mud generated during the same period [4]. Despite ongoing research, red mud currently has few commercially viable paths to beneficial re-use. It is estimated that very little of red mud is beneficially re-used worldwide [13-14]. Selle asemel, the red mud is pumped from the alumina refinery into storage impoundments or landfills, where it is stored and monitored at large cost [3]. Seetõttu, both an economic and environmental argument can be made for improving the quality of bauxite prior to refining, in particular if such improvement can be done through low-energy physical separation techniques.
While proven reserves of bauxite are expected to last for many years, the quality of the reserves that can be economically accessed is declining [1,3]. For refiners, who are in the business of processing bauxite to make alumina, and eventually aluminum metal, this is a challenge with both financial and environmental implications
Dry methods such as electrostatic separation may be of interest of the bauxite industry for the pre-concentration of bauxite prior to the Bayer process. Electrostatic separation methods that utilize contact, või tribo-elektriline, charging is particularity interesting because of their potential to separate a wide variety of mixtures containing conductive, isoleerimine, ja pooljuhtivad osakesed. Tribo-elektriline laadimine toimub diskreetsuse korral, erinevad osakesed põrkuvad üksteisega, või kolmanda pinnaga, mille tulemuseks on pinnalaengu erinevus kahe osakese tüübi vahel. Laenguerinevuse märk ja suurus sõltub osaliselt elektronide afiinsuse erinevusest (või tööfunktsioon) osakeste tüüpide vahel. Eraldamine on võimalik väliselt rakendatava elektrivälja abil.
Seda tehnikat on tööstuslikult kasutatud vertikaalsetes vabalangemise tüüpi eraldajates.. Vabalangemise eraldajates, osakesed omandavad kõigepealt laengu, then fall by gravity through a device with opposing electrodes that apply a strong electric field to deflect the trajectory of the particles according to sign and magnitude of their surface charge [18]. Free-fall separators can be effective for coarse particles but are not effective at handling particles finer than about 0.075 et 0.1 mm [19-20]. One of the most promising new developments in dry mineral separations is the tribo-electrostatic belt separator. See tehnoloogia on laiendatud osakeste suuruse vahemik, et peenemad osakesed kui tavalise elektrostaatilise eraldamise tehnika, vahemikku, kus on ainult ujuvad edukalt varem.
Tribo-electrostatic separation utilizes electrical charge differences between materials produced by surface contact or triboelectric charging. In simplistic ways, when two materials are in contact, the material with a higher affinity for electros gains electrons thus changes negative, samal ajal väiksemate electron afiinsus teenustasude positiivne materjal.
ST seadmed & Tehnoloogia (STET) tribo-electrostatic belt separator offers a novel beneficiation route to pre-concentrate bauxite ores. The STET dry separation process offers bauxite producers or bauxite refiners an opportunity to perform pre-Bayer-process upgrading of bauxite ore to improve the quality. This approach has many benefits, Sealhulgas: Reduction in operating cost of refinery due to lower consumption of caustic soda by reducing input reactive silica; savings in energy during refining due to lower volume of inert oxides (Fe2O3, TiO2, Non-reactive SiO2) entering with bauxite; smaller mass flow of bauxite to refinery and therefore less energy requirement to heat and pressurize; reduction in red mud generation volume (st, red mud to alumina ratio) by removing reactive silica and inert oxide; ja, tighter control over input bauxite quality which reduces process upsets and allows refiners to target ideal reactive silica level to maximize impurity rejection. Improved quality control over bauxite feed to refinery also maximizes uptime and productivity. Peale selle, reduction in red mud volume translates into less treatment and disposal costs and better utilization of existing landfills.
The preprocessing of bauxite ore prior to the Bayer process may offer significant advantages in terms of processing and sales of tailings. Unlike red mud, tailings from a dry electrostatic process contain no chemicals and do not represent a long-term environmental storage liability. Unlike red mud, dry by-products/tailings from a bauxite pre-processing operation can be utilized in cement manufacture as there is no requirement to remove the sodium, which is detrimental to cement manufacture. In fact – bauxite is already a common raw material for Portland cement manufacturing. Extending operating life of existing bauxite mines may also be reached by improving quarry utilization and maximizing recovery.
2.0 Experimental
2.1 Materials
STET conducted pre-feasibility studies in over 15 different bauxite samples from different locations around the world using a bench-scale separator. Neist, 7 different samples were
Tabel 2. Result of chemical analysis bauxite samples.
2.2 Methods
Experiments were conducted using a bench-scale tribo-electrostatic belt separator, hereafter referred as ‘benchtop separator’. Bench-scale testing is the first phase of a three-phase technology implementation process (See Table 3) including bench-scale evaluation, pilot-scale testing and commercial-scale implementation.
Benchtop eraldajana kasutatakse tõendite tribo-elektrostaatiline maksustamise ja kas materjal on hea kandidaat elektrostaatilise beneficiation skriining. Tabelis on toodud peamised erinevused iga seade 3. Kuigi igas faasis kasutatavad seadmed erinevad suurus, operatsiooni põhimõte on põhimõtteliselt sama.
Tabel 3. Kolmefaasilise rakendamisprotsessi STET tribo-elektrostaatiline vöö eraldaja tehnoloogia abil
| Phase | Used for: | Electrode Length cm | Type of Process |
|---|---|---|---|
| 1- Bench Scale Evaluation | Qualitative Evaluation | 250 | Batch |
| 2- Pilot Scale Testimine | Quantitative evaluation | 610 | Batch |
| 3- Commercial Scale Implementation | Commercial Production | 610 | Continuous |
Nagu näha tabelis 3, Peamine erinevus benchtop eraldaja ja pilot-scale ja kommertstasandil eraldusjooned on benchtop eraldaja pikkus on ligikaudu 0.4 pilot-scale ja kommertstasandil pikkus korda. Kuna eraldaja efektiivsus on elektroodi pikkuse funktsioon, pinkide skaala testimist ei saa kasutada pilootskaalas testimise asendajana. Katseprojekt on vajalik, et teha kindlaks eraldamise ulatus, mida STET-protsess võib saavutada, ja teha kindlaks, kas STET-protsess suudab täita tooteeesmärgid antud söödamäärade alusel. Selle asemel, pinkide eraldajat kasutatakse selleks, et välistada kandidaatmaterjalid, mis tõenäoliselt ei näita olulist eraldamist pilootskaala tasandil. Pingil saadud tulemused ei ole optimeeritud, ja täheldatud eraldamine on väiksem kui see, mida täheldataks kaubandusliku suurusega STET eraldajal.
Testing at the pilot plant is necessary prior to commercial scale deployment, aga, testing at the bench-scale is encouraged as the first phase of the implementation process for any given material. Peale selle, in cases in which material availability is limited, the benchtop separator provides a useful tool for the screening of potential successful projects (st, projects in which customer and industry quality targets can be met using STET technology).
2.2.1 STET Triboelectrostatic Belt Separator
Aastal tribo-elektrostaatiline vöö eraldaja (Joonis 1 ja joonis 2), materjal söödetakse õhuke vahe 0.9 – 1.5 cm kahe paralleelse tasapinnalised elektroodide vahel. Osakeste triboelectrically tuleb maksta interparticle kontakt. Näiteks, in the case of a bauxite sample which main constituents are gibssite, kaolinite and quartz mineral particles, the positively charged (gibssite) and the negatively charged (kaolinite and quartz) on huvitatud vastas elektroodid. Seejärel pühitakse osakesed pideva liikuva avatud võrgusilma vööga ja edastatakse vastassuunas.. Vöö liigub kõrval iga elektroodi poole vastupidine otsad eraldaja osakesed. Elektriväli vajab ainult osakeste liigutamist vaid väikese osa sentimeetrist, et liigutada osakest vasakult liikuvast voolust paremale liikuvale voolule.. The counter current flow of the separating particles and continual triboelectric charging by particle collisions provides for a multi-stage separation and results in excellent purity and recovery in a single-pass unit. Selle kõrge kiirus võimaldab väga kõrge läbilase, kuni 40 tonni tunnis ühe eraldaja. Kontrollides erinevaid protsessi parameetrid, the device allows for optimization of mineral grade and recovery.
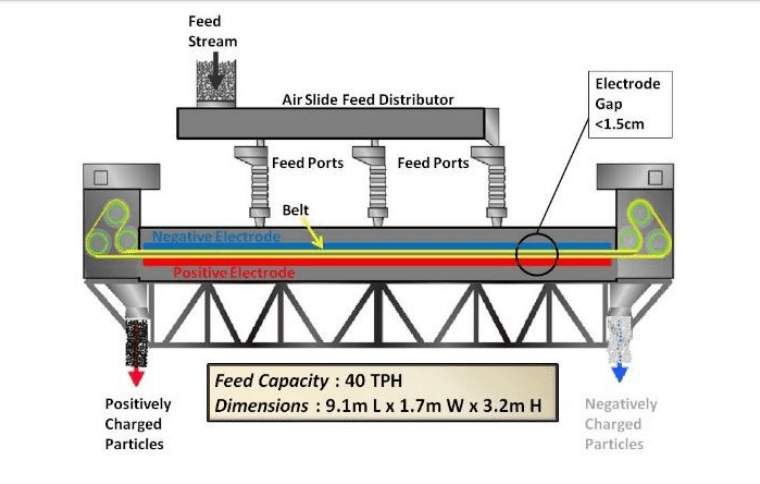
Joonis 1. Triboelectric vöö eraldaja skemaatiline
Eraldaja on suhteliselt lihtne. Vöö ja nendega seotud rullid on ainus liikuvate osade. Elektroodid on paigal ja koosneb asjakohaselt vastupidav materjal. Vöö on valmistatud plastmassist. Eraldaja elektroodi pikkus on ligikaudu 6 meetrit (20 jalga.) ja laiuse 1.25 meetrit (4 jalga.) jaoks täissuuruses äripinnaga. Tarbitav võimsus on alla 2 kilovatt-tund tonni materjali kohta, mida töödeldakse suurema osa võimsusest, mida tarbivad kaks turvavööd sõitvat mootorit.
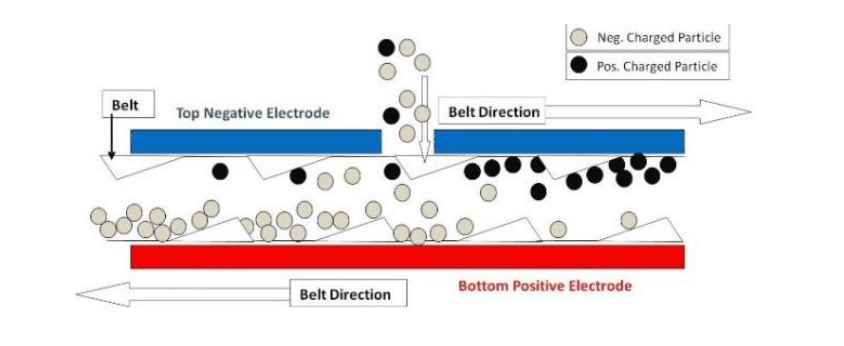
Joonis 2. Eraldamine tsooni detail
Protsess on täiesti kuiv, ei ole täiendavaid materjale nõuab ja toodab mingit vee või õhuga emissiooni. For mineral separations the separator provides a technology to reduce water usage, pikendada reserv eluiga ja/või taastada ja ümber töödelda sabasid.
Süsteemi kompaktsus võimaldab paindlikkust paigalduskonstruktsioonides. The tribo-electrostatic belt separation technology is robust and industrially proven and was first applied industrially to the processing of coal combustion fly ash in 1997. Tehnoloogia on efektiivne süsinikuosakeste eraldamisel söe mittetäielikust põlemisest, klaasjast alumiiniumoksiidi mineraalosakestest lendtuhas. Tehnoloogia on aidanud kaasa mineraalirikka lendtuha taaskasutamisele tsemendiasendajana betooni tootmisel..
Alates 1995, üle 20 million tonnes of product fly ash have been processed by the STET separators installed in the USA. Lendtuha eraldamise tööstusajalugu on loetletud tabelis 4.
In minerals processing, the triboelectric belt separator technology has been used to separate a wide range of materials including calcite/quartz, Talk/magnesiit, barüüdi/jahvatatakse.

Joonis 3. Commercial tribo-electrostatic belt separator
Tabel 4. Tribo-elektrostaatilise vöö eraldamise tööstuslik kasutamine lendtuha jaoks.
| Kasulikkus / elektrijaam | Asukoht | Äritegevuse alustamine | Rajatise üksikasjad |
|---|---|---|---|
| Duke Energy – Roxboro jaam | Põhja-Carolina USA | 1997 | 2 Eraldajad |
| Talen Energy- Brandon Shores | Maryland USA | 1999 | 2 Eraldajad |
| Scottish Power- Longanneti jaam | Šotimaa Suurbritannia | 2002 | 1 Eraldaja |
| Jacksonville Electric-St. Johns Riveri elektripark | Florida USA | 2003 | 2 Eraldajad |
| Lõuna-Mississippi elektrienergia -R.D. Hommik | Mississippi USA | 2005 | 1 Eraldaja |
| Uus Braunschweigi Power-Belledune | Uus Brunswick Kanada | 2005 | 1 Eraldaja |
| RWE npower-Didcot Station | Inglismaa Suurbritannia | 2005 | 1 Eraldaja |
| Talen Energy-Brunneri saare jaam | Pennsylvania USA | 2006 | 2 Eraldajad |
| Tampa elektri-suur Kurvi jaam | Florida USA | 2008 | 3 Eraldajad |
| RWE npower-Aberthaw jaam | Wales UK | 2008 | 1 Eraldaja |
| EDF Energy-West Burtoni jaam | Inglismaa Suurbritannia | 2008 | 1 Eraldaja |
| ZGP (Lafarge tsement /Ciech Janikosoda JV) | Poola | 2010 | 1 Eraldaja |
| Korea Kagu-Jõud- Yeongheung | Lõuna-Korea | 2014 | 1 Eraldaja |
| PGNiG Termika-Sierkirki | Poola | 2018 | 1 Eraldaja |
| Taiheiyo Tsemendifirma-Chichibu | Jaapan | 2018 | 1 Eraldaja |
| Armstrong Fly Ash- Eagle Cement | Filipiinid | 2019 | 1 Eraldaja |
| Korea Kagu-Jõud- Samcheonpo | Lõuna-Korea | 2019 | 1 Eraldaja |
2.2.2 Bench-scale testing
Standard process trials were performed around the specific goal to increase Al_2 O_3 concentration and to reduce the concentration of gangue minerals. Tests were conducted on the benchtop separator under batch conditions, with testing performed in duplicate to simulate steady state, and ensure that any possible carryover effect from the previous condition was not considered. Prior to each test, a small feed sub-sample was collected (designated as ‘Feed’). Upon setting all operation variables, the material was fed into the benchtop separator using an electric vibratory feeder through the center of the benchtop separator. Samples were collected at the end of each experiment and the weights of product end 1 (designated as ‘E1’) and product end 2 (designated as ‘E2’) were determined using a legal-for-trade counting scale. For bauxite samples, ‘E2’ corresponds to the bauxite-rich product. For each set of sub-samples (st, Feed, E1 and E2) LOI, main oxides composition by XRF, reactive silica and available alumina was determined. XRD characterization was performed on selected sub-samples.
3.0 Results and Discussion
3.1. Samples Mineralogy
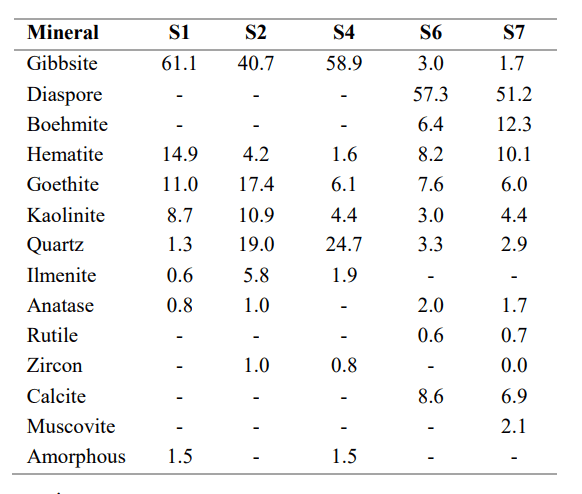
Results of the quantitative XRD analyses for feed samples are included in Table 5. The majority of the samples were primarily composed of gibbsite and varying amounts of goethite, hematite, kaoliniidist, and quartz. Ilmenite and anatase were also evident in minor amounts in the majority of the samples.
There was a change in the mineral composition for S6 and S7 as these feed samples were primarily composed of diaspore with minor amounts of calcite, hematite, götiit, boehmite, kaoliniidist, gibbsite, kvarts, anatase, and rutile being detected. An amorphous phase was also detected in S1 and S4 and ranged from approximately 1 et 2 percent. This was probably due to either the presence of a smectite mineral, or non-crystalline material. Since this material could not be directly measured, results for these samples should be considered approximate.
3.2 Bench-scale experiments
A series of test runs were performed on each mineral sample aimed at maximizing Al2O3 and decreasing SiO_2 content. Species concentrating to the bauxite-rich product will be indicative of positive charging behavior. Results are shown in Table 6
Tabel 5. XRD analysis of feed samples.
Tabel 6. Summary Results.
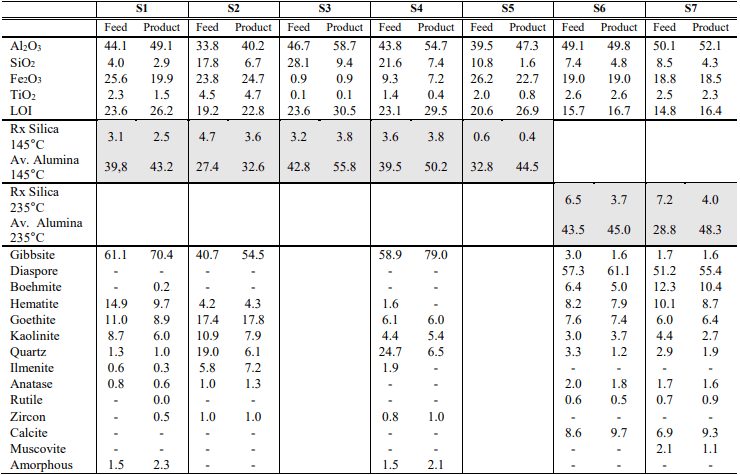
Testing with the STET benchtop separator demonstrated significant movement of Al2O3 for all samples. Separation of Al2O3 was observed for S1-5 which were mainly gibbsite, and also for S6-7 which were mainly diaspore. Lisaks, Fe2O3 teised olulised elemendid, SiO2 ja TiO2 näitasid enamikul juhtudel märkimisväärset liikumist. For all samples, süüteka kadude liikumine (LOI) Al2O3 järgiv liikumine. In terms of reactive silica and available alumina, for S1-5 which are nearly all gibbsite (aluminum trihydrate) values should be considered at 145°C while for S6-7 for which the dominant mineral is diaspore (aluminum monohydrate) values should be assessed at 235°C. For all samples testing with the STET benchtop separator demonstrated a substantial increase in available alumina and a significant reduction in reactive silica to product for both trihydrate and monohydrate bauxite samples. Movement of major mineral species was also observed and is graphically shown below in Figure 4.
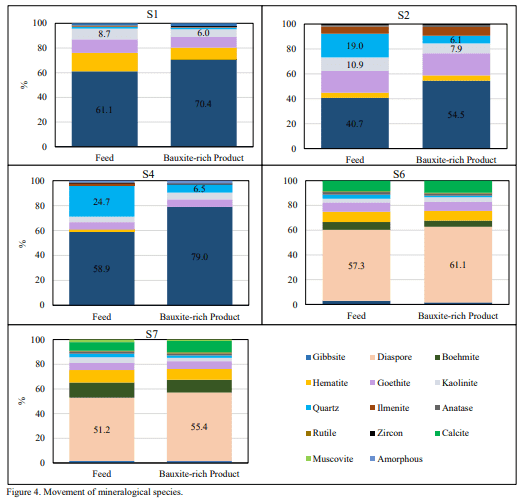
In terms of mineralogy, STET benchtop separator demonstrated concentration of the alumina bearing species gibbsite and diaspore to the bauxite-rich product while simultaneously rejecting other gangue species. Arvud 5 ja 6 show selectivity of mineral phases to the bauxite-rich product for trihydrate and monohydrate samples, respectively. Selectivity was calculated as the difference between the mass deportment to product for each mineral species and the overall mass recovery to product. A positive selectivity is indicative of mineral concentration to the bauxite-rich product, and of an overall positive charging behavior. Contrary, a negative selectivity value is indicative of concentration to the bauxite-lean coproduct, and of an overall negative charging behavior.
For all trihydrate low-temperature samples (st, S1, S2 and S4) kaolinite exhibited a negative charging behavior and concentrated to the bauxite-lean co-product while gibbsite concentrated to the bauxite-rich product (Joonis 5). For all monohydrate high-temperature samples (st, S6 and S7) both reactive silica bearing minerals, kaolinite and quartz, exhibited a negative charging behavior. For the latter, diaspore and boehmite reported to the bauxite-rich product and exhibited a positive charging behavior (Joonis 6).
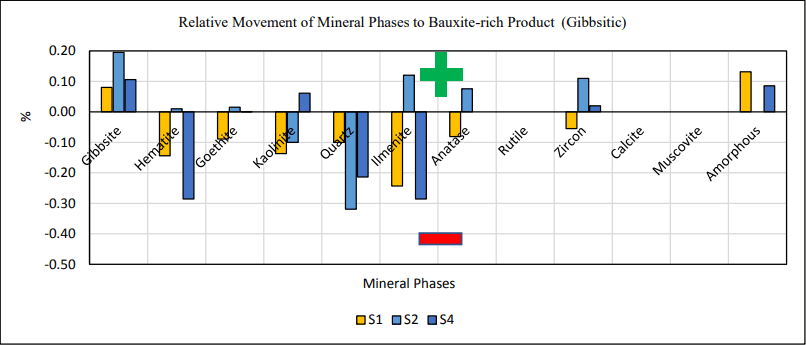
Joonis 5. Selectivity of mineral phases to product.
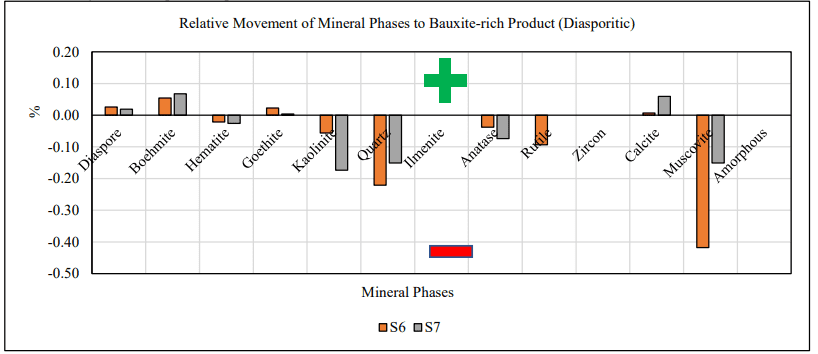
Joonis 6. Selectivity of mineral phases to product.
Measurements of available alumina and reactive silica demonstrate substantial movement. For low temperature bauxites (S1-S5), the amount of reactive silica present per unit of available alumina was reduced from 10-50% on a relative basis (Joonis 7). A similar reduction was observed in the high temperature bauxites (S6-S7) as can be seen in Figure 7.
The bauxite to alumina ratio was calculated as the inverse of the available alumina. The bauxite to alumina ratio was decreased by between 8 – 26% in relative terms for all samples tested (Joonis 8). This is meaningful as it represents an equivalent reduction in mass flow of bauxite that needs to be fed to the Bayer process.
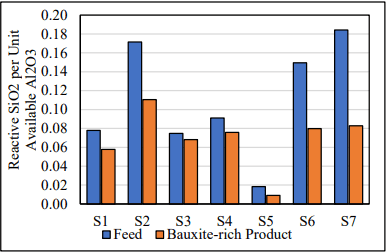
Joonis 7. Reactive SiO2 per unit of Available Al2O3
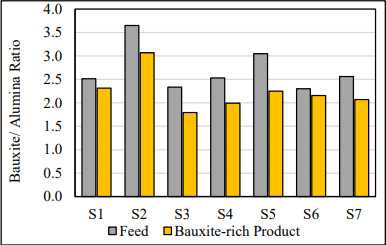
Joonis 8. Bauxite to Alumina ratio.
3.3 Arutelu
The experimental data demonstrates that the STET separator increased available Al2O3 while simultaneously reducing SiO_2 content. Joonis 9 presents a conceptual diagram of the expected benefits associated to the reduction of reactive silica and the increase of available alumina prior to the Bayer Process. The authors calculate that the financial benefit to an alumina refiner would be in the range of $15-30 USD per ton of alumina product. This reflects avoided cost from caustic soda lost to de-silicaton product (DSP), energy savings from reducing the input of bauxite to the refinery, reduction in red mud generation and a small revenue stream generated from selling the low-grade bauxite by-product to cement producers. Joonis 9 outlines the expected benefits of implementing STET triboelectrostatic technology as a mean to pre-concentrate bauxite ore prior the Bayer process.
Installation of the STET separation process for bauxite pre-processing could be performed either at the alumina refinery or the bauxite mine itself. Aga, the STET process requires dry grinding of the bauxite ores prior to separation, to liberate the gangue, therefore the logistics of grinding and processing the bauxite at the refinery may be more straightforward.
As one option – the dry bauxite would be ground using well-established dry grinding technology, for example a vertical roller mill or impact mill. The finely ground bauxite would be separated by the STET process, with the high-alumina bauxite product sent to the alumina refinery. The installation of dry grinding would allow for the elimination of wet grinding traditionally used during the Bayer process. It is assumed that the operating cost of dry grinding would be roughly comparable to the operating cost of wet grinding, especially considering the wet grinding performed today is performed on a highly alkaline mixture, leading to considerable maintenance costs.
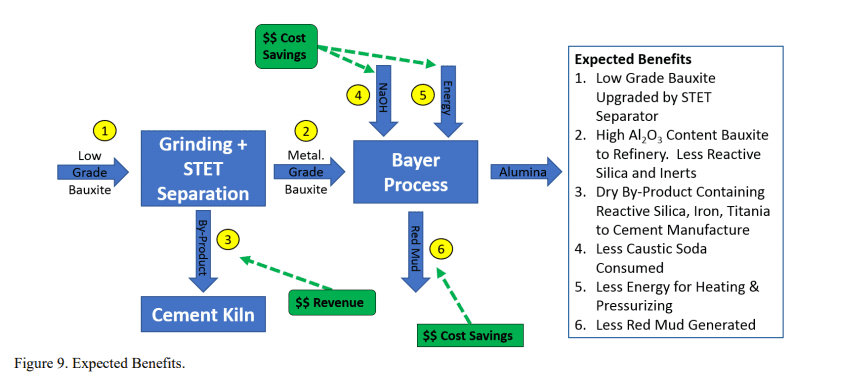
The dry low-grade bauxite co-product (tailings) from the separation process would be sold to cement manufacture as an alumina source. Bauxite is commonly added to cement manufacture, and the dry co-product, unlike red mud, does not contain sodium which would prevent its use in cement manufacture. This provides the refinery with a method of valorizing material that would otherwise exit the refining process as red mud, and would require long term storage, representing a cost.
An operating cost calculation performed by the authors estimates a project benefit of $27 USD per ton of alumina, with the major impacts achieved through reduction in caustic soda, reduction in red mud, valorization of the co-product and fuel savings due to lower volume of bauxite to the refinery. Therefore an 800,000 ton per year refinery could expect a financial benefit of $21 M USD per year (See Figure 10). This analysis does not consider potential savings from reducing import or logistics costs of bauxite, which may further enhance the project return.
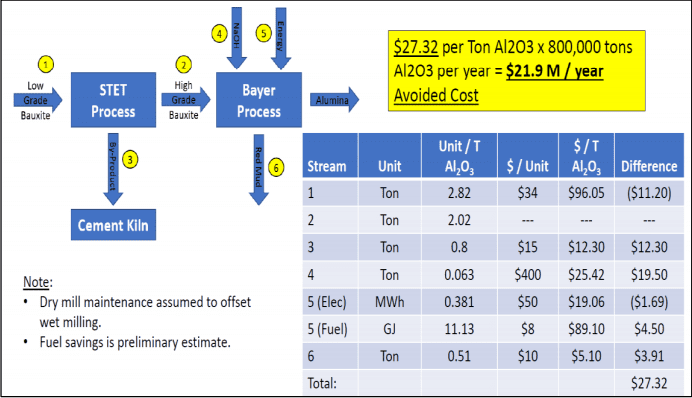
Joonis 10. Benefits of Reactive Silica Reduction and Available Alumina increase.
4.0 Conclusions
In summary, dry processing with the STET separator offers opportunities to generate value for bauxite producers and refiners. The pre-processing of bauxite prior to refining will reduce chemical costs, lower the volume of red mud generated and minimize process upsets. STET technology could allow bauxite processors to turn non-metallurgical grade into metallurgical grade bauxite – which could reduce need for imported bauxite and/or extend exiting quarry resource life. STET process could also be implemented to generate higher quality non-metallurgical grade and metallurgical grade bauxite, and cement grade bauxite by-products prior to the Bayer process.
The STET process requires little pre-treatment of the mineral and operates at high capacity – up to 40 tones per hour. Energy consumption is less than 2 kilowatt-hours per ton of material processed. Peale selle, the STET process is a fully commercialized technology in minerals processing, and therefore does not require the development of new technology.
Viited
1. Bergsdal, Håvard, Anders H. Strømman, and Edgar G. Hertwich (2004), “The aluminium industry-environment, technology and production”.
2. Das, Subodh K., and Weimin Yin (2007), “The worldwide aluminum economy: The current state of the industry” JOM 59.11, PP. 57-63.
3. Vincent G. Hill & Errol D. Sehnke (2006), “Bauxite”, in Industrial Minerals & Rocks: Kaupade, Turgudel, and Uses, Society for Mining, Metallurgy and Exploration Inc., Englewood, CO, PP. 227-261.
4. Evans, Ken (2016), “The history, challenges, and new developments in the management and use of bauxite residue”, Journal of Sustainable Metallurgy 2.4, PP. 316-331
5. Gendron, Robin S., Mats Ingulstad, and Espen Storli (2013), “Aluminum ore: the political economy of the global bauxite industry”, UBC Press.
6. Hose, H. R. (2016), “Bauxite mineralogy”, Essential Readings in Light Metals, Springer, Cham, PP. 21-29.
7. Authier-Martin, Monique, et al. (2001),”The mineralogy of bauxite for producing smelter-grade alumina”, JOM 53.12, PP. 36-40.
8. Hill, V. G., and R. J. Robson (2016), “The classification of bauxites from the Bayer plant standpoint”, Essential Readings in Light Metals, Springer, Cham, PP. 30-36.
9. Songqing, Gu (2016). “Chinese Bauxite and Its Influences on Alumina Production in China”, Essential Readings in Light Metals, Springer, Cham, PP. 43-47.
10. Habashi, Fathi (2016) “A Hundred Years of the Bayer Process for Alumina Production” Essential Readings in Light Metals, Springer, Cham, PP. 85-93.
11. Adamson, A. N., E. J. Bloore, and A. R. Carr (2016) “Basic principles of Bayer process design”, Essential Readings in Light Metals, Springer, Cham, PP. 100-117.
12. Anich, Ivan, et al. (2016), “The Alumina Technology Roadmap”, Essential Readings in Light Metals. Springer, Cham, PP. 94-99.
13. Liu, Wanchao, et al. (2014), “Environmental assessment, management and utilization of red mud in China”, Puhtama tootmise ajakiri 84, PP. 606-610.
14. Evans, Ken (2016), “The history, challenges, and new developments in the management and use of bauxite residue”, Journal of Sustainable Metallurgy 2.4, PP. 316-331.
15. Liu, Yong, Chuxia Lin, and Yonggui Wu (2007), “Characterization of red mud derived from a combined Bayer Process and bauxite calcination method”, Journal of Hazardous materials 146.1-2, PP. 255-261.
16. U.S. Geological Survey (USGS) (2018), “Bauxite and Alumina”, in Bauxite and Alumina Statistics and information.
17. Paramguru, R. K., P. C. Rath, and V. N. Misra (2004), “Trends in red mud utilization–a review”, Mineral Processing & Extractive Metall. Rev. 2, PP. 1-29.
18. Manouchehri, H, Hanumantha Roa, K, & Forssberg, K (2000), “Review of Electrical Separation Methods, Osa 1: Aluspõhimõtteid, Mineraalid & Metallurgical Processing”, Vol. 17, Ei. 1, lk 23–36.
19. Manouchehri, H, Hanumantha Roa, K, & Forssberg, K (2000), “Review of Electrical Separation Methods, Osa 2: Praktilised kaalutlused, Mineraalid & Metallurgical Processing”, Vol. 17, Ei. 1, lk 139–166.
20. Ralston O. (1961), Segatud granuleeritud tahkete ainete elektrostaatiline eraldamine, Elsevier Kirjastus, välja prinditud.