Select Language:
ST Equipment & Technology LLC has developed a process that removes ammonia from fly ash. The process recovers 100% of the fly ash treated and the resulting ash meets all specifications for use in Concrete. STET’s ammonia removal process can be used alone or in combination with the company’s carbon separation technology. The carbon separation process is not affected by the presence of ammonia on the fly ash. This modular approach offers the lowest cost solution for treating otherwise unusable fly ash…
Download PDFNeedham Technical Center
Removing Ammonia from Fly Ash
J. Bittner, S. Gasiorowski, and F. Hrach
Separation Technologies, LLC 101 Hampton Avenue, Needham, Massachusetts, USA
Abstract
The supply of fly ash available for use as a pozzolan in concrete may be severely impacted by the effects of air quality regulations on utility plant operations. Specifically, mandated reductions in NOx, particulate, and SO3 containing aerosol emission levels are expected to require the installation of control systems which may use ammonia as a reagent. Depending on the level of ammonia present in the flue gas at the unit precipitators, the collected fly ash may be heavily contaminated with ammonia primarily as ammonium sulfate salts.
ST Equipment & Technology LLC (STET) has developed a process that removes ammonia from fly ash. The process recovers 100% of the fly ash treated and the resulting ash meets all specifications for use in concrete. ST’s ammonia removal process can be used alone or in combination with the company’s carbon separation technology. The carbon separation process is not affected by the presence of ammonia on the fly ash. This modular approach offers the lowest cost solution for treating otherwise unusable fly ash.
The ST ammonia removal system is operating at two locations in the USA and one in Europe. The ammonia levels of the untreated fly ash have varied between 200 and 3000 mg NH3 / kg ash (part per million by mass, or ppm). The ST process has successfully reduced the ash ammonia level to less than 50 mg NH3 / kg ash. Over 500,000 tons of fly ash has been sold to ready mix concrete producers from ST’s ammonia removal process.
Ammonia Contaminated Fly Ash
The supply of fly ash available for use as a pozzolan in concrete may be severely impacted by the effects of air quality regulations on utility plant operations.1 Specifically, mandated reductions in NOx , particulate, and SO3 containing aerosol emission levels are expected to require the installation of control systems which may use ammonia as a reagent. Depending on the level of ammonia present in the flue gas at the electrostatic precipitators, the collected fly ash may be heavily contaminated with ammonia primarily as ammonium sulfate salts 1,2. For NOx control, the flue gas ammonia level will be set by the amount of ammonia “slip‿, i.e. unreacted ammonia present after the SCR or SNCR unit. To reduce particulate or SO3 aerosol emissions, ammonia is injected into the flue gas prior to the precipitators and will be deposited on the fly ash. The degree to which this occurs is dependent on the SO3 content, fly ash sulfur content, alkalinity of the fly ash, the ammonia concentration, and ash loading in the flue gas.
The use of fly ash in concrete requires that the fly ash have specific physical and chemical properties3. The pozzolanic properties of the ash are activated in the concrete by the generation of highly alkaline free lime from hydration of the cement. When fly ash contains ammonia, this ammonia is liberated as a gas by the action of the highly alkaline solution of the concrete. The alkalinity shifts the equilibrium of ammonium ion in solution to molecular ammonia according to the following equation:
NH4+(aq) + OH–(aq) ↔ NH3(aq) + H2O
The dissolved molecular ammonia is easily released from solution as free ammonia gas.
ST Equipment & Technology LLC 101 Hampton Avenue Needham, MA 02494 Tel: 781-972-2700
Needham Technical Center
Ammonia is a strong smelling compound that carries the connotation of barnyards, manure and urine. A strong odor of ammonia is unacceptable to the concrete producer, the contractor working with the concrete, and the ultimate concrete user.4
The finished properties of the concrete are not adversely affected when using ammonia contaminated fly ash, but the odor is unacceptable, particularly if the concrete is used in underground or enclosed spaces.5,6,7 Depending on the specifics of the location, including the amount of fresh air circulation, ammonia odor was found not to be objectionable when using fly ashes containing 100 to 200 mg NH3 / kg (part per million by mass, or ppm). In order to assure that no problems are encountered, the ammonia content of fly ash should be no greater than 100 ppm2,8.
However, the addition of ammonia at the power generation plant can result in fly ash ammonia contents of 200 to 2500 ppm, rendering the fly ash unacceptable for use in concrete.8 Thus, reducing air quality problems by controlling the air emissions of power plants increases a solid waste disposal problem and increases CO2 greenhouse emissions by increasing the amount of cement used in concrete production. Removal of ammonia from fly ash so that it can be used in concrete would benefit the utility by avoiding solid waste disposal, the concrete producer, user, and ultimate owner by lowering the cost of materials and increasing product quality, and the environment by reducing emissions of greenhouse gases from cement production.
The amount of ammonia and ammonium salts found in fly ash is related to the amount of ammonia present in the flue gas. For a typical pulverized coal unit using eastern bituminous coal with a high heating value of 12,000 BTU/lb (6667 kcal/kg), the flue gas to coal mass ratio is approximately 8 to 9.5. When the ash content of the coal is 10%, and 80% of the ash in the coal is recovered as fly ash (20% to bottom ash), the flue gas / ash ratio is approximately 100. If all of the ammonia species present in the flue gas were deposited or adsorbed on the fly ash, the concentration of the ammonia in the ash would be approximately 50 times greater than in the flue gas on a mass basis, e.g. an ammonia “slip” of 2 ppm by volume would result in an ash containing 100 mg / kg (ppm by wt.) ammonia. Actual measurements show this relationship to be correct.9 The concentration of ammonia on fly ash will vary among operating units dependent on the ash content of the coal and the fly ash to bottom ash ratio as well as other factors.
Ammonia injection to electrostatic precipitators (ESP) to improve efficiency and reduce plume opacity can result in very high levels of ammonia in fly ash. Levels up to 2500 ppm ammonia in ash have been found for such systems. SNCR operations typically operate with ammonia slip concentrations of 5 to 20 ppm, with ash contaminated to a level of 200 to 1000 ppm ammonia. SCR systems generally are designed to operate at maximum ammonia slip levels of 2 or 5 ppm, depending on the specifics of the installation.10 Generally, the greater the NH3/NOx ratio, the greater the NOx reduction which will be obtained, with a higher ammonia slip resulting. However, ammonia slip greater than 2 ppm may result in ash-ammonia contents of greater than 100 ppm, producing an unmarketable ash.11 Many power plants in Japan and Germany operating SCR units designed for a 2 ppm maximum slip have seen little impact on the marketability of fly ash.1,5 However, some SCR’s around the world have been designed for 5 ppm ammonia slip. Operation of these units at this high slip level will most likely result in greater than 100 ppm of ammonia on the fly ash.
Controlling ammonia slip to low levels also reduces plugging and corrosion of downstream equipment due to deposition of ammonium sulfates.1,10 Avoiding air preheater plugging due to this phenomenon is expected to be even more important to unit operators when burning high sulfur, US coals. Thus, management of ammonia slip to minimize unit outage time will be a greater concern than controlling the ammonia level of fly ash. The result
ST Equipment & Technology LLC 101 Hampton Avenue Needham, MA 02494 Tel: 781-972-2700
Needham Technical Center
may be that no significant problem develops for the utilization of ash at some utility sites, depending on the specific design and operation of the emission control systems.
The STET Process
STET has developed a process that removes ammonia from fly ash.12 The process recovers 100% of the fly ash treated and the resulting ash meets all specifications for use in concrete. STET’s ammonia removal process can be used alone or in combination with the company’s carbon separation technology. The carbon separation process is not affected by the presence of ammonia. This modular approach offers the lowest cost solution for treating otherwise unusable fly ash.
To remove ammonia as a gas from the fly ash, the STET process utilizes the same fundamental chemical reaction that results in ammonia release in concrete. Liberation of ammonia from fly ash requires that the ammonium ion
–molecular ammonia equilibrium be shifted in favor of ammonia by the presence of alkali. Fly ashes with naturally high alkalinity need no additional alkali. For less alkaline ashes, any strong alkali will serve. The cheapest source of alkali is lime (CaO). The reaction of ammonium salts with lime liberating ammonia is strongly favored by chemical equilibrium. The chemical reaction occurs rapidly once the compounds are dissolved.
The overall reaction can be generalized as:
(NH4)2SO4(s) + CaO(s) → 2NH3(g) + CaSO4(s) + H2O(g)
However, the vapor pressures of the solids are quite low and the reaction cannot occur in the gas or solid phase. Ammonium sulfate is highly soluble in water and dissociates to ammonium ions and sulfate ions.
(NH4)2SO4(aq) → 2NH4+(aq) + SO42-(aq)
Lime is highly unstable on exposure to water, favoring a highly exothermic reaction commonly known as “slaking”, producing calcium hydroxide or hydrated lime.
CaOs +H2O → Ca(OH)2(s)
The hydrated lime is only sparingly soluble in water, producing calcium and hydroxide ions.
Ca(OH)2(s) ↔ Ca2+(aq) + 2 OH–(aq)
Calcium sulfate is also sparingly soluble, so as calcium ions are made available by dissolving the hydrated lime, they are primarily consumed by precipitation of calcium sulfate.
Ca2+(aq) + SO42-(aq) → CaSO4(s)
Finally, an equilibrium exists between ammonium ions and ammonia dissolved in water.
NH4+(aq) + OH–(aq) ↔ NH3(aq) + H2O
ST Equipment & Technology LLC 101 Hampton Avenue Needham, MA 02494 Tel: 781-972-2700
Needham Technical Center
The degree to which ammonium ions are converted to molecular ammonia is dependent on the pH of the aqueous system, higher pH’s favoring the formation of molecular ammonia. This equilibrium is well known.13
A key feature of the STET process is the use of a minimum quantity of water (1 to 4%, typically 2%) and minimal quantities of alkali (< 2%). Large amounts of water are detrimental to the process, slowing the rate of ammonia release. The pH of the resulting ash / lime / water mixture should be greater than pH 10.0. Very small quantities of alkali are added to assure this pH, depending on the natural pH of the ash. Typically, less than 1% Ca(OH)2 is required, even with fly ash with naturally acidic pH. Calcium based alkalis are used which result in minimal alteration of the fly ash chemistry. The process is performed at ambient temperature.
Since the cost of the alkali and cost of drying the product are major operating expenses and sizing the mixing and drying equipment are the major components of capital cost, carefully measuring the reagent requirements for a specific ash is necessary to optimize the economics of the process.
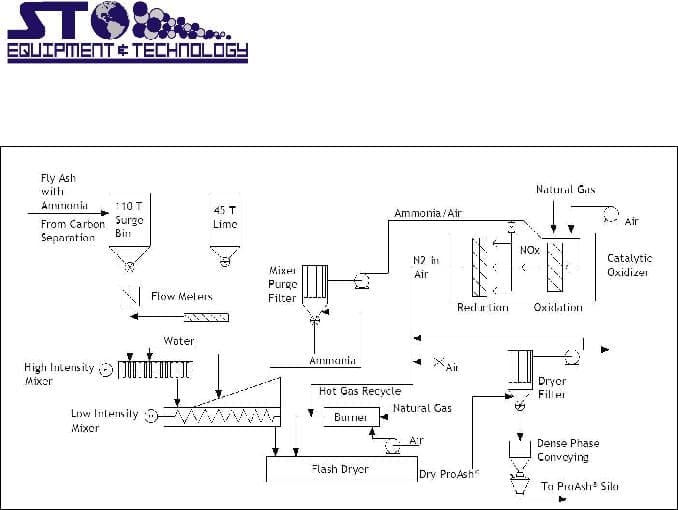
The process flow diagram for the continuous operation of the STET process is presented in Figure 1. Ash, water and alkali in controlled proportions are metered to a mixer. To assure rapid mixing and uniform dispersion of the added water and alkali, a high intensity mixer is used. The residence time in this type of mixer is very low, on the order of one second. Ammonia is immediately released but complete evolution requires mixing times of 3 to 4 minutes to allow for mass transport of the gas from the bulk of the ash to allow for mass transport of the gas from the bulk of the ash.
To obtain this mixing time and assure good transport of the ammonia from the bulk of the ash, a low intensity device such as a pug mill is used as a secondary mixer. Since the moisture content of the ash is very low, the material flows through this mixer as a highly agitated dry powder. Ammonia gas collected in both the high and low speed mixers is either recycled to the generating unit flue or converted to nitrogen in a two- stage catalytic unit.
ST Equipment & Technology LLC 101 Hampton Avenue Needham, MA 02494 Tel: 781-972-2700
Needham Technical Center
Figure 1: STET Ammonia Removal Process Flow Diagram
The deammoniated ash is dried by conveying the material through a flash drier to remove excess water. Due to the minimal amount of water added, water consumed in the formation of hydrated calcium sulfate upon reaction with soluble sulfate in the ash, and loss of water during the low intensity mixing stage, only a small amount of water needs to be removed by the drier. This minimizes the energy demand of the drying stage. Final ash temperatures of approximately 150oF are adequate to produce a completely free-flowing, product fly ash with moisture contents well below the ASTM C 618 specification of 3 wt. %.3
The first full-scale application of STET’s ammonia removal process began operating in 2003 at ST’s ash processing facility at the Jacksonville Electric Authority St. Johns River Power Park in Jacksonville, Florida. This commercial scale operation handles up to 40 tons per hour of contaminated ash, reducing the ammonia content to less than 30 ppm. Ammonia levels in the incoming fly ash varies from ~200 to 900 ppm. The process is very robust, resulting in 90+% ammonia removal under all trial settings, producing ash well below our target of maximum 50 ppm ammonia. Final moisture contents are <0.3%. Representative results are listed in Table 2. Over 250,000 tons of ammonia-contaminated ash has been successfully processed at the Jacksonville facility.
ST Equipment & Technology LLC 101 Hampton Avenue Needham, MA 02494 Tel: 781-972-2700
Needham Technical Center
Table 2: Typical commercial scale ammonia system results
|
Feed Rate, |
Initial Ammonia, |
Final Ammonia, |
Lime rate, |
Water rate, |
|
tons/hr |
ppm |
ppm |
% of feed |
% of feed |
|
21 |
910 |
25 |
1.35 |
1.9 |
|
25 |
190 |
9 |
0.88 |
2.6 |
|
40 |
350 |
12 |
0.70 |
2.05 |
|
18 |
242 |
20 |
0.82 |
1.52 |
Fly ash at Big Bend Station in Tampa, Florida is contaminated with ammonia due to the injection of ammonia into the power plant’s ESP systems to control the emission of SO3 aerosol generated by the SCR NOx control system. At other power plants, ammonia contamination also occurs with SNCR NOx control and the use of ammonia to enhance particle collection in ESP systems. At Big Bend, the fly ash ammonia levels for units with ammonia injection range from 750 to 3360 ppm ammonia. Consequently, the fly ash intended for concrete production must be treated by the ST ammonia removal process.
The design of the STET ammonia removal process installed at Big Bend (Figure 2) is the second full scale installation and includes many refinements. Material handling equipment is improved to allow operation at higher rates. The drying system is downsized from the previous design and includes recycling of hot gases to reduce the overall energy requirements of the system. Lastly, the ammonia released in the process is fed to a two-stage catalytic unit where the collected ammonia gas is converted into nitrogen. The heat generated by this reaction is recovered and used to supplement the energy requirement of the fly ash flash drying system. The use of the two-stage catalytic system results in minimal NOx emissions. The process recovers 100% of the fly ash treated and the resulting ash meets all specifications for use in concrete.
This commercial scale operation can handle up to 52 tons per hour of contaminated ash, reducing the ammonia content to less than 75 mg/kg. The process is very robust, resulting in 97+% ammonia removal, producing ash well below our target of maximum 100 mg/kg ammonia. Final moisture contents are <0.5%.
An STET ammonia removal process has been in operation at the RWE npower Aberthaw station ash processing facilities in the UK since 2008. Aberthaw adds ammonia to improve ESP collection efficiency with ammonia levels on the ash typical 200 ppm and the process is designed for 500 ppm maximum.
ST Equipment & Technology LLC 101 Hampton Avenue Needham, MA 02494 Tel: 781-972-2700

Needham Technical Center
Figure 2: Ammonia removal system at Big Bend Station, Tampa Florida
The Product Ash
The low ammonia fly ash product meets all chemical and physical requirements of the ASTM C 618 standard and resulting concrete properties are identical to ash not subjected to the ammonia removal process. Table I compares typical properties of ash from one generating station along with properties of the plant ash reduced in ammonia content from 250 mg / kg to 20 mg / kg by the continuous ST process. Note that the range of calcium oxide content observed for the ash from this source is 1.4 to 12%. The addition of up to 1% Ca(OH)2 (0.75% as CaO) will result in only minor changes in the ash chemistry, broadening the variability insignificantly.
Concrete testing performed on the deammoniated ash showed it to be an excellent pozzolanic material. Compressive strength development using this ash is as good as or better than unammoniated ash from this source. Long term concrete durability properties are also excellent, including improved corrosion resistance, sulfate resistance, chloride permeability, and lime shrinkage.
ST Equipment & Technology LLC 101 Hampton Avenue Needham, MA 02494 Tel: 781-972-2700
Needham Technical Center
Table I: Fly Ash Physical and Chemical Properties.
|
|
ASTM C 618 specification |
Plant # 1 Ash Properties, Typical |
Plant # 1 Ash After Ammonia Removal |
|
|
Chemical Composition |
|
|
|
|
|
Silicon Dioxide |
– |
55 – 61 % |
59.8 % |
|
|
Aluminum Oxide |
– |
15 – 25 % |
23.8 % |
|
|
Iron Oxide |
– |
5 – 10.9% |
7.35 |
|
|
Total (SiO2 + Al2O3 + Fe2O3) |
70.0 Min. |
78 – 91 % |
90.9 % |
|
|
Sulfur Trioxide |
5.0 Max |
0.13 – 1.4% |
0.87% |
|
|
Calcium Oxide |
– |
1.4 – 12 % |
1.79 % |
|
|
Moisture Content |
3.0 Max. |
0.0 – 0.3 % |
0.21% |
|
|
Loss on Ignition |
6.0 Max. |
0.7 – 2.6 % |
0.91% |
|
|
Sodium Oxide |
– |
0.1 – 0.7 |
0.14% |
|
|
Potassium Oxide |
– |
0.5 – 2.2% |
0.56% |
|
|
Available Alkalis (as Na2O) |
1.5 % Max |
0.5 – 0.8% |
0.51% |
|
|
Physical Test Results |
|
|
|
|
|
Fineness, retained on #325 sieve |
34% Max. |
8 – 16% |
14% |
|
|
Strength Activity Index |
|
|
|
|
|
Ratio to Control @ 7 days |
– |
81 – 95 % |
94.8% |
|
|
Ratio to Control @ 28 days |
75% Min. |
94 – 102% |
99.5% |
|
|
Water Requirement, % of Control |
105% Max. |
93 – 97% |
94.2% |
|
|
Soundness, Autoclave Expansion |
0.8% Max. |
-0.035 – 0.010 |
-0.033 |
|
|
Dry Shrinkage, Increase @ 28 Day |
0.03% Max. |
-0.01 – 0.010 |
-0.009 |
|
|
Density |
– |
2.35 – 2.45 |
2.36 |
|
Summary
The degree to which good quality fly ash will be compromised as the result of NOx or particulate emission systems in the future will depend upon technology choices made by utilities to reduce NOx, particulate matter emissions and plume opacity. However, STET’s ammonia removal process can be utilized to remove the ammonia from contaminated ash, producing a high value material for use in concrete production. Recovered ammonia can be recycled to the generating unit for reutilization. This ammonia removal process can be installed as a stand-alone system or can be used in conjunction with STET’s successful fly ash carbon removal system.
1Stewart, B.R., Unintended Effects of EPA’s Recent Ozone Transport Rule, Proceedings, 1999 Conference on Selective Catalytic and Non-Catalytic Reduction for NOx control, May 1999, pp.9-10.
2Sloss, L.L., Hjalmarsson, A-K, Soud, H.N., Campbell, L.M., Stone, D.K., Shareef, G.S., Emmel, T., Maibodi, M., Livengood, C.D., Markussen, J. Nitrogen Oxides Control Technology Fact Book, Noyes Data Corporation, pp. 94-95, 1992
3“Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use as a Mineral Admixture in Concrete”, ASTM C 618, American Society for Testing and Materials, Philadelphia, PA.
4Majors, R.K., Hill, R., McMurry, R., Thomas, S., A Study of the Impact of Ammonia Injection on Marketable Fly Ash including Quality Control Procedures, Proceedings, 1999 Conference on Selective Catalytic and Non-Catalytic Reduction for NOx control, May 1999, pp.11-13.
5Van der Brugghen, F.W., Gast, C.H., Van den Berg, J.W., Kuiper, W.H., Visser, R., Problems encountered During the Use of Ammonium contaminated Fly Ash. Proceedings: EPRI / EPA 1995 Joint Symposium on Station Combustion NOx Control, May 16-19, 1995. Book 4, Session 8A, pp. 1-16.
6Van den Berg, J.W., Cornelissen, H.A.W. ,Effect of low NOx Technologies on Fly Ash Quality, Proceedings: 13th International Symposium on Use and Management of Coal Combustion Products, 1999, pp. 29-1 – 29-11.
7Koch, H-J., Prenzel, H., Tests on Odour Developments in the Casting of a Concrete Screed – Using a NH3-Contaminated Fly Ash, Concrete Precasting Plant and Technology, Vol 11, 1989 pp. 72-75.
8Fisher, B.C., Blackstock, T. Fly Ash Beneficiation using an Ammonia Stripping Process, 12th International Symposium on Coal Combustion By-Products Management and Use, 1997 pp. 65-1 – 65-8.
ST Equipment & Technology LLC 101 Hampton Avenue Needham, MA 02494 Tel: 781-972-2700
Needham Technical Center
9Larrimore, L., Dodgen, D., Monroe, L., Characterization of Ammonia Effects on Ash and Evaluation of Removal Methods, Proceedings: 13th International Symposium on Use and Management of Coal Combustion Products, 1999, pp. 16-1 – 16-15.
10Control of Nitrogen Oxide Emissions: Selective Catalytic Reduction (SCR), Clean Coal Technology, Topical Report Number 9. U.S. Department of Energy and Southern Company Services, Inc. July, 1997.
11O’Connor, D., Larrimore, L, Dodgen, D., Monroe, L., The Effects of Ammonia-Based NOx Reduction on Fly Ash: Ammonia Adsorption on Ash, Proceedings, EPRI-DOE-EPA Combined Utility Air Pollution Control Symposium: The MEGA Symposium, August, 1999, paper # 16.
12Gasiorowski, S.A., and Hrach, F.J., Method for Removing Ammonia from Ammonia Contaminated Fly Ash, United States Patent Number 6,077,494, June 20, 2000.
13Thurston, R.V., Russo, R.C., Emerson, K., Aqueous Ammonia Equilibrium – Tabulation of Percent Un-ionized Ammonia, United States Environmental Protection, EPA-600/3-79-091, August, 1979.
ST Equipment & Technology LLC 101 Hampton Avenue Needham, MA 02494 Tel: 781-972-2700